General Principles And Processes of Isolation Of Elements
In the extraction of copper from its sulphide ore, the metal finally obtained by the reduction of cuprous oxide with
-
Iron (II) sulphide
-
Carbon monoxide
-
Copper (I) sulphide
-
Sulphur dioxide
C.
Copper (I) sulphide
Reduction of Copper oxide done with Copper (I) sulphide.
Cu2S + 2Cu2O → 6Cu +SO2 ↑
Sponsor Area
Some More Questions From General Principles And Processes of Isolation Of Elements Chapter
Which of the ores mentioned in Table 6.1. could be concentrated by magnetic separation method?
Table 6.1 Principal ores of some important metal.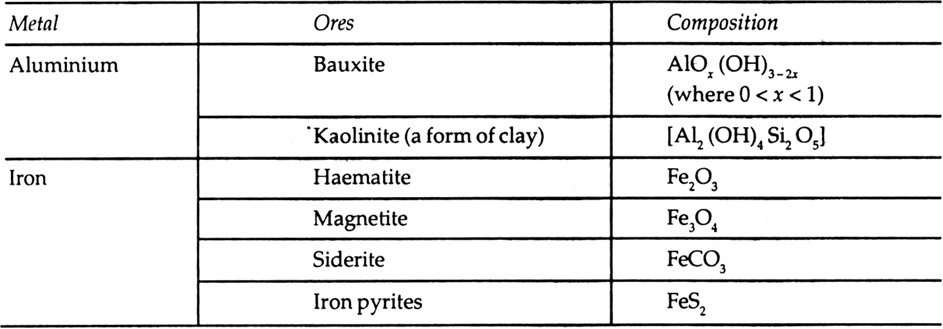

What is the significance of leaching in the extraction of aluminium?
Is it true that under certain conditions. Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
What is the role of depressant in froth floatation process?
Titanium is a metal of modern times. It is obtained as oxide. Why it could not be extracted earlier despite its wide occurence?
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present?
What criterion is followed for the selection of the stationary phase in chromatography?
What is the role of cryolite in the metallurgy of aluminium?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area