Aldehydes, Ketones and Carboxylic Acids
(a) How will you convert the following?
(i) Propanone to Propan-2-ol
(ii) Ethanal to 2-hydroxy propanoic acid
(iii) Toluene to benzoic acid
(b) Give simple chemical test to distinguish between:
(i) Pentan-2-one and Pentan-3-one
(ii) Ethanal and Propanal
(a)
(i) Propanone to Propan-2-ol
![]()
(ii) Ethanal to 2-Hydroxypropanoic acid
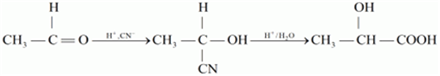
(iii) Toluene to benzoic acid.

(b)
(i) Pentan-2-one and Pentan-3-one
By Iodoform Test
Pentan-2-one being a methyl ketone when treated with NaOI (I2/NaOH) gives yellow precipitate of iodoform but pentan-3-one does not.
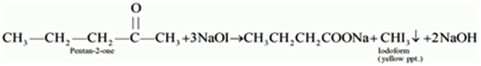

(ii) Ethanal and Propanal
![]()
By Iodoform test
Ethanal containing group CH3-C=O linked to H, reacts with I2/NaOH (or NaOI) to give yellow precipitate of iodoform but propanal does not contain group CH3-C=O linked to H or C and hence does not react with I2/NaOH to give yellow precipitate.


Sponsor Area
Some More Questions From Aldehydes, Ketones and Carboxylic Acids Chapter
Write the structures of products of the following reactions:
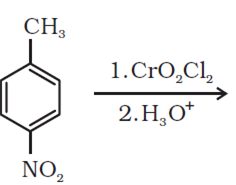
Arrange the following compounds in increasing order of their boiling points:
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions:
Ethanal, Propanal, Propanone, Butanone.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions:
Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Predict the products of the following reactions:
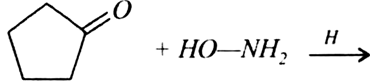
Predict the products of the following reactions:
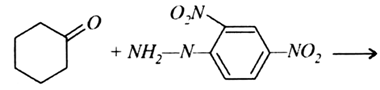
Predict the products of the following reactions:

Predict the products of the following reactions:
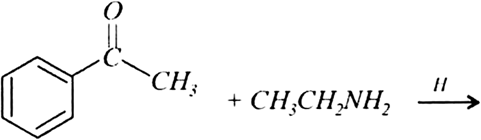
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area