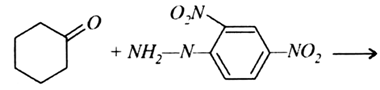
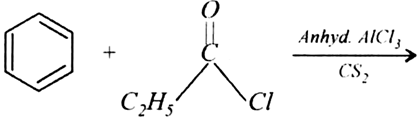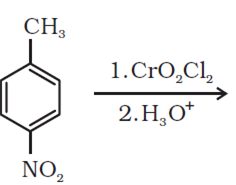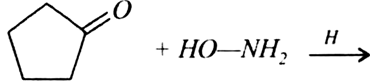Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions:
Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.

The +I effect is more in ketone than in aldehyde. Hence, acetphenone is the least reactive in nucleophilic addition reaction. Among aldehyde, the +l effects is the highest in p-tolualdehyde because of the presence of the electron donating –CH3 group and the lowest in p-nitro bezaldehyde because of the presence of the electron withdrawing –NO2 group. Hence, the increasing order of the reactivates of the given as;
Acetophenone < p-Tolualdehyde < Benzaldehyde < p-Nitrobenzaldehyde.