General Principles And Processes of Isolation Of Elements
Which solution is used for the leaching of silver metal in the presence of air in the metallurgy of silver?
Dilute solution of NaCN and KCN is used for leaching of silver metal in the presence of air in the metallurgy of silver, for example in the following reaction
AgS + 4NaCN ⇌ 2Na[Ag(CN)2] + Na2SAgS + 4NaCN ⇌ 2Na[Ag(CN)2] + Na2S
The solution of sodium Argento cyanide combines with zinc dust and forms sodium tetra cyanozicate and precipitated silver. This precipitated silver is called spongy silver.
Zn+2Na[Ag(CN)2] → Na2[Zn(CN)4] + 2AgZn + 2Na[Ag(CN)2] → Na2[Zn(CN)4] + 2Ag
The spongy silver is fused with potassium nitrate to obtain pure silver. Then the silver obtained is purified by an electrolytic process.
Sponsor Area
Some More Questions From General Principles And Processes of Isolation Of Elements Chapter
Which of the ores mentioned in Table 6.1. could be concentrated by magnetic separation method?
Table 6.1 Principal ores of some important metal.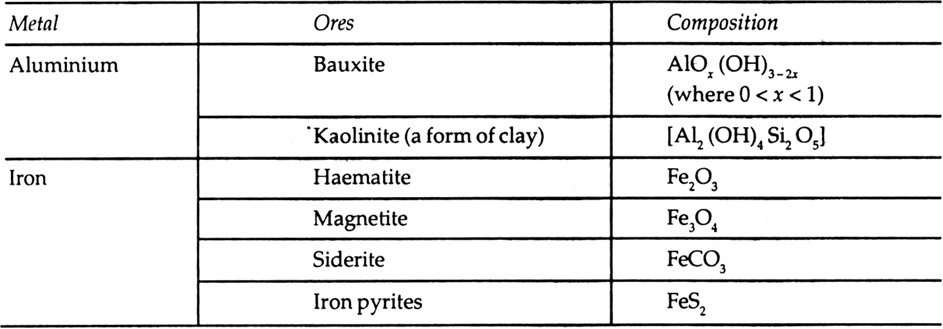

What is the significance of leaching in the extraction of aluminium?
Is it true that under certain conditions. Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
What is the role of depressant in froth floatation process?
Titanium is a metal of modern times. It is obtained as oxide. Why it could not be extracted earlier despite its wide occurence?
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present?
What criterion is followed for the selection of the stationary phase in chromatography?
What is the role of cryolite in the metallurgy of aluminium?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area