Haloalkanes and Haloarenes
Give reasons:
n-Butyl bromide has higher boiling point than t-butyl bromide.
The boiling point of n-butyl bromide is higher than that of t-butyl bromide because n-butyl bromide is a straight chain molecule having larger surface area and therefore, has stronger intermolecular forces. On the other hand, t-butyl bromide is branched molecule, so it has a smaller surface area. Hence, it has weaker intermolecular force. Thus, n- Butyl bromide has higher boiling point than t-butyl bromide.
Sponsor Area
Some More Questions From Haloalkanes and Haloarenes Chapter
Write structures of the following compounds:
1-Bromo-4-sec butyl-2-methyl benzene.
Why is sulphuric acid not used during the reaction of alcohols with KI?
Write structures of different dihalogen derivatives of propane.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i) A single monochloride.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i)Three isomeric monochlorides.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
Four isomeric monochlorides.
Draw the structures of major monohalo products in each of the following reactions:![]()
Draw the structures of major monohalo products in each of the following reactions: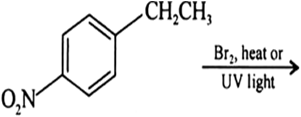
Draw the structures of major monohalo products in each of the following reactions: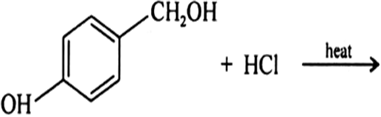
Draw the structures of major monohalo products in each of the following reactions: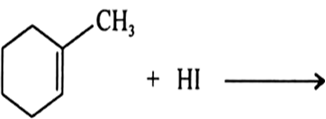
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area