General Principles And Processes of Isolation Of Elements
State briefly the principles which serve as basis for the following operations in metallurgy:
(i) Froth floation process.
(ii) Zone refinnting.
(iii) Refining by liquation.
(i) Froth floatation process: This method has been in use for removing gangue from sulphide ores. In this process, a suspension of the powdered ore is made with water. To it, collectors and froth stabilizers are added. Collectors (e.g., pine oils, fatty acids, xanthates etc.) enhance non-wettability of the mineral particles and froth stabilizers (e.g., cresols, aniline) stabilizes the froth.
Fig. Froth floatation process.
The mineral particles become wet by oils while the gangue particles by water. A roasting paddle agitates the mixture and draws air in it. As a result, froth is formed which carries the mineral particles. The froth is light and is skimmed off. It is then dried for recovery of the ore particles. Sometimes, it is possible to separate two sulphide ores by adjusting proportion of oil to water or using ‘depressants’. For example, in case of an ore containing ZnS and Pb, the depressant used is NaCN. It selectively prevents ZnS from coming to the froth but allows PbS to come with the froth.
(ii) Zone refining: This method is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal. A circular mobile heater is fixed at one end of a rod of impure metal. The molten zone moves along with the heater which is moved forward. As the heater moves forward, the pure metal crystallizes out the melt and the impurities pass on into the adjacent molten zone.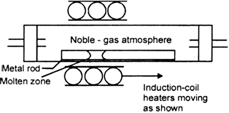
Fig. Zone Refining Process.
The process is repeated several times and the heater is moved in the same direction. At one end, impurities get concentrated. This end is cut off. This method is very useful for producing semiconductor and other metals of very high purity e.g., germanium, silicon, boron, gallium and indium.
(iii) Liquation: Liquation, technique for separating constituents of an ore, a metal, or an alloy by partial melting. When the material is heated to a temperture where one of the constituents melts and the other remains solid, the liquid constituent can be drained off. It was formerly used for extracting antimony minerals from ore and for separating silver from copper with the use of lead as a solvent. It is still used in some refining of tin
Sponsor Area
Some More Questions From General Principles And Processes of Isolation Of Elements Chapter
Is it true that under certain conditions. Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
What is the role of depressant in froth floatation process?
Titanium is a metal of modern times. It is obtained as oxide. Why it could not be extracted earlier despite its wide occurence?
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present?
What criterion is followed for the selection of the stationary phase in chromatography?
What is the role of cryolite in the metallurgy of aluminium?
Why is zinc not extracted from zinc oxide through reduction using CO?
What is the role of graphite rod in the electrometallurgy of aluminium?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area