General Principles And Processes of Isolation Of Elements
Why is the extraction of copper from pyrites difficult than from its oxide ore through reduction.
In the graph of ΔrG° Vs T for the formation of oxides, the Cu2O line is almost at the top. So it is quite easier to reduce oxide ores of copper directly to the metal by heating with coke (both the lines of C, CO and C, CO2 are at much lower positions in the graph particularly after 500-600 K).
However sulphide ores are roasted/smelted to give oxides.
2Cu2S + 3O2 → 2Cu2O + 2SO2
The oxide can then be easily reduced to metallic copper using coke.
Cu2O + C → 2Cu + CO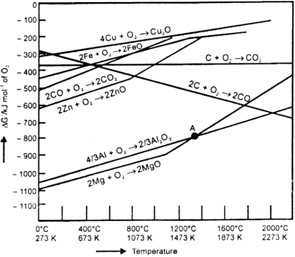
Sponsor Area
Some More Questions From General Principles And Processes of Isolation Of Elements Chapter
What is the role of depressant in froth floatation process?
Titanium is a metal of modern times. It is obtained as oxide. Why it could not be extracted earlier despite its wide occurence?
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present?
What criterion is followed for the selection of the stationary phase in chromatography?
What is the role of cryolite in the metallurgy of aluminium?
Why is zinc not extracted from zinc oxide through reduction using CO?
What is the role of graphite rod in the electrometallurgy of aluminium?
Predict conditions under which Al might be expected to reduce MgO.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area