Aldehydes, Ketones and Carboxylic Acids
Sponsor Area
Some More Questions From Aldehydes, Ketones and Carboxylic Acids Chapter
Write the structures of products of the following reactions:
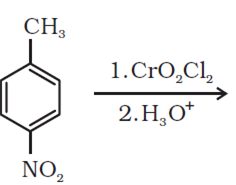
Arrange the following compounds in increasing order of their boiling points:
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions:
Ethanal, Propanal, Propanone, Butanone.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions:
Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Predict the products of the following reactions:
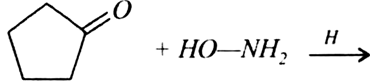
Predict the products of the following reactions:
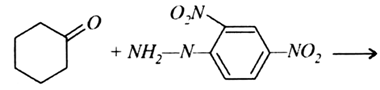
Predict the products of the following reactions:

Predict the products of the following reactions:
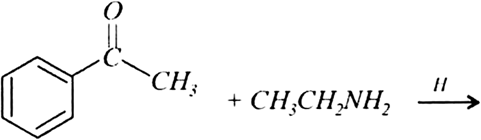
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area