The p-Block Elements
Question
NF3 does not have donor properties like ammonia. Explain.
Answer
NF3 has a pyramidal shape with one lone-pair on N atom.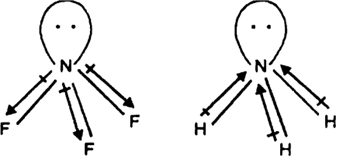
The lone-pair on N is in opposite direction to the N—F bond moments and therefore it has very low dipole moment (about 0.234 D). Thus it does not show donor properties. But ammonia has high dipole moment because its lone pair is in the same direction as the N—H bond moments. Thus it has donor properties.
The electronegtivity also influence the donar properties. fluorine is more electronegtive than hydrogen thus fluroine pull electron from nitrogen but hydrogen is not.
Sponsor Area
Some More Questions From The p-Block Elements Chapter
What is the covalency of nitrogen in N2O5?
Bond angle in PH4+ is higher than that in PH3. Why?
Write a balanced equation for the hydrolytic reactions PCl5 in heavy water.
What is the basicity of H3PO4?
What happens when H3PO3 is heated?
List the important source of sulphur.
Write the order of thermal stability of the hydrides of Group 16 elements.
Why is H2O a liquid and H2S a gas?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area