Haloalkanes and Haloarenes
Give reasons:
Vinyl chloride is hydrolysed more slowly than ethyl chloride.
Vinyl chloride is hydrolyzed more slowly than ethyl chloride because the C-Cl bond in vinyl chloride has is sp2 hybridized thus, C-Cl in vinyl chloride has more s character from the C- atom, therefore the bond is stronger and more difficult to break.
The C-Cl bond in vinyl chloride has some double bond and have ability to delocalized the electron to over the molecule. Thus, vinyl chloride is hydrolysed more slowly than ethyl chloride.
Sponsor Area
Some More Questions From Haloalkanes and Haloarenes Chapter
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
Four isomeric monochlorides.
Draw the structures of major monohalo products in each of the following reactions:![]()
Draw the structures of major monohalo products in each of the following reactions: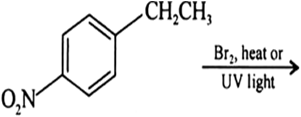
Draw the structures of major monohalo products in each of the following reactions: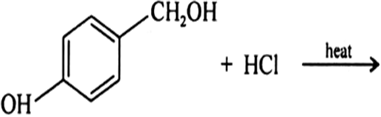
Draw the structures of major monohalo products in each of the following reactions: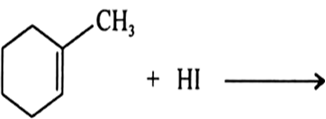
Draw the structures of major monohalo products in each of the following reactions: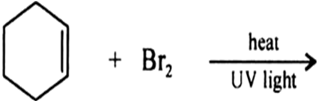
Arrange each set of compounds in order of increasing boiling points:
Bromomethane, Bromoform, Chloro-methane, Dibromomethane.
Arrange each set of compounds in order of increasing boiling points:
1-chloropropane, Isopropyl chloride, 1-chlorobutane.
Arrange each set of compounds in order of increasing boiling points:![]()
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area