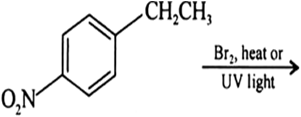
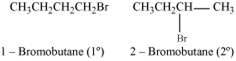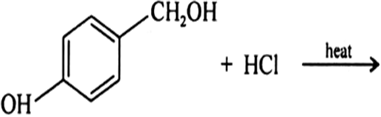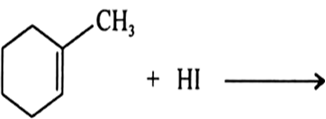Arrange each set of compounds in order of increasing boiling points:![]()
1-bromobutane is a primary alkyl halide whereas 2-bromobutane is secondary alkyl halide. The nucleophile approaching is more hindered in 2- bromobutane than in 1-bromobutane. Therefore, 1- bromobutane reacts more rapidly than 2- bromobutane by an SN2 mechainsm.