Haloalkanes and Haloarenes
Explain giving reason although haloalkanes are polar in character, yet they are insoluble in water.
Alkyl halides are polar in nature due to difference electronegativity between C-atom of alkyl group and halogen atom. However, they are insoluble in water, because alkyl halides cannot form hydrogen bonds with water moleules. Also, they cannnt break the hydrogen bond that already existing among the water molecules.
Sponsor Area
Some More Questions From Haloalkanes and Haloarenes Chapter
Write structures of the following compounds:
1-Bromo-4-sec butyl-2-methyl benzene.
Why is sulphuric acid not used during the reaction of alcohols with KI?
Write structures of different dihalogen derivatives of propane.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i) A single monochloride.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i)Three isomeric monochlorides.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
Four isomeric monochlorides.
Draw the structures of major monohalo products in each of the following reactions:![]()
Draw the structures of major monohalo products in each of the following reactions: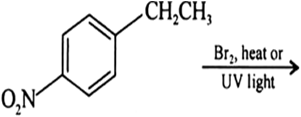
Draw the structures of major monohalo products in each of the following reactions: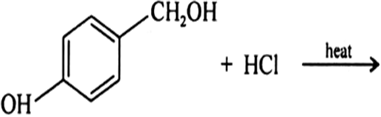
Draw the structures of major monohalo products in each of the following reactions: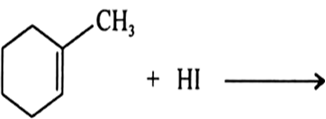
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area