Haloalkanes and Haloarenes
Explain why an alkyl halide (or methyl chloride) is hydrolysed more readily than aryl halide (or chlorobenzene).
Due to high electronegativity of halogen atom (say chlorine atom), the —C—X (or Cl—Cl) bond in alkyl halide (or methyl chloride) is slightly polar and carbon atom of—C—X (or —C—Cl) bond acquires a slight positive charge. Thus
The Cδ+ atom is easily attacked by nucleophile (electron-rich species), such as OH–. Thus hydrolysis takes place easily.
On the other hand, aryl halide (or chlorobenzene) is resonance stabilized, so the C—Cl bond has a partial double character. Consequently, the rupture of this partial double bond becomes rather difficult. Hence, aryl halide (or chlorobenzene) is less reactive than alkyl halide (or chloromethane). Moreover, the C—Cl bond in aryl halide has a sp2hybridized C-atom, while it is sp3 hybridized in alkyl halide (or chloromethane). Since sp3 hybrid C-atom is more electronegative than the sp3 hybrid C-atom, so C–Cl bond in aryl halide (or chlorobenzene) has less tendency to release electrons to Cl-atom. In other words, the C—Cl bond in aryl halide (or chlorobenzene) is less polar than in alkyl halide (or chloromethane). This makes nucleophilic substitution (say by OH– group) more difficult. Hence, alkyl halide (or methyl chloride) is hydrolysed more readily than aryl halide (or chlorobenzene).
Sponsor Area
Some More Questions From Haloalkanes and Haloarenes Chapter
Write structures of the following compounds:
1, 4-Dibromo but-2-ene
Write structures of the following compounds:
1-Bromo-4-sec butyl-2-methyl benzene.
Why is sulphuric acid not used during the reaction of alcohols with KI?
Write structures of different dihalogen derivatives of propane.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i) A single monochloride.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i)Three isomeric monochlorides.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
Four isomeric monochlorides.
Draw the structures of major monohalo products in each of the following reactions:![]()
Draw the structures of major monohalo products in each of the following reactions: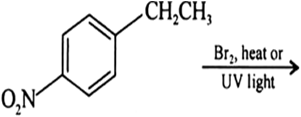
Draw the structures of major monohalo products in each of the following reactions: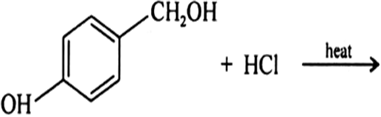
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area