Haloalkanes and Haloarenes
Arrange the following in order of increasing boiling points:
Alcohol have ability to form hydrogen bond therefore it forms bond with hydrogen and thus strengthen the bond. thus, boiling point of alcohol is more then haloalkane.
Since molecule (iii) has two hydroxy group therefore it forms more stronger hydrogen bond than (iv). In case of haloalkane bromine is larger in size thus vander waal forces is more as campare to chlorine thus order of increasing boiling point is given in order;
Sponsor Area
Some More Questions From Haloalkanes and Haloarenes Chapter
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
Four isomeric monochlorides.
Draw the structures of major monohalo products in each of the following reactions:![]()
Draw the structures of major monohalo products in each of the following reactions: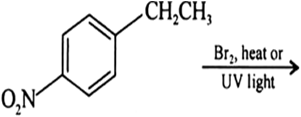
Draw the structures of major monohalo products in each of the following reactions: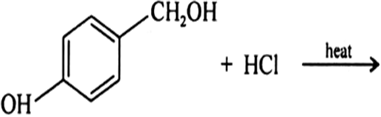
Draw the structures of major monohalo products in each of the following reactions: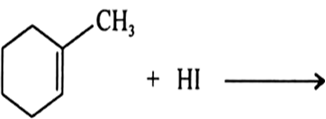
Draw the structures of major monohalo products in each of the following reactions: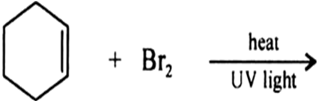
Arrange each set of compounds in order of increasing boiling points:
Bromomethane, Bromoform, Chloro-methane, Dibromomethane.
Arrange each set of compounds in order of increasing boiling points:
1-chloropropane, Isopropyl chloride, 1-chlorobutane.
Arrange each set of compounds in order of increasing boiling points:![]()
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area