Haloalkanes and Haloarenes
p-Dichlorobenzene has higher melting point and solubility than those of o- and m-isomer. Discuss.
The melting point of p-isomer is quite higher than ortho and meta isomers. This is due to the fact that it has symmetrical structure and therefore its molecules can be easily packed closely in crystal lattice. As a result, intermolecular forces of attraction are stronger and therefore greater energy is required to break its lattice and it melts at higher temperature.
Sponsor Area
Some More Questions From Haloalkanes and Haloarenes Chapter
Write structures of different dihalogen derivatives of propane.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i) A single monochloride.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i)Three isomeric monochlorides.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
Four isomeric monochlorides.
Draw the structures of major monohalo products in each of the following reactions:![]()
Draw the structures of major monohalo products in each of the following reactions: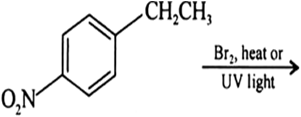
Draw the structures of major monohalo products in each of the following reactions: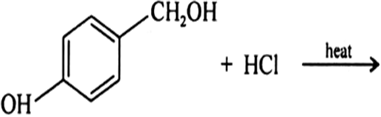
Draw the structures of major monohalo products in each of the following reactions: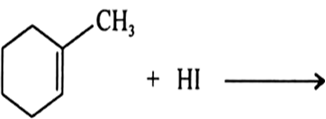
Draw the structures of major monohalo products in each of the following reactions: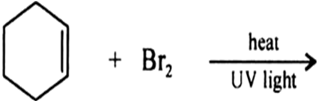
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area