Haloalkanes and Haloarenes
What are ambident nucleophiles? Explain with an example.
Ambident nucleophiles: The nucleophiles with two nucleophilic centres are called ambident nucleophile.For example cyanide act as ambident nucleophilies.
There are two nucleophiles centres, at carbon and nitrogen in cyanide ion (C ≡ N:). They can react through either of these centres. Depending on the reagent and the reaction conditions, the reaction may take place predominantly at one of these centres.
Examples are C ≡ N: (reagent KCN, NaCN) etc.
Sponsor Area
Some More Questions From Haloalkanes and Haloarenes Chapter
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i)Three isomeric monochlorides.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
Four isomeric monochlorides.
Draw the structures of major monohalo products in each of the following reactions:![]()
Draw the structures of major monohalo products in each of the following reactions: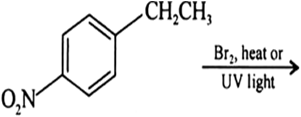
Draw the structures of major monohalo products in each of the following reactions: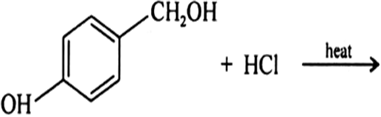
Draw the structures of major monohalo products in each of the following reactions: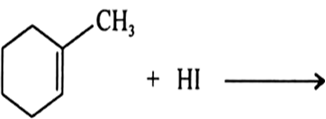
Draw the structures of major monohalo products in each of the following reactions: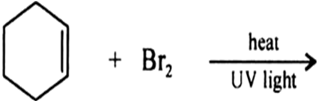
Arrange each set of compounds in order of increasing boiling points:
Bromomethane, Bromoform, Chloro-methane, Dibromomethane.
Arrange each set of compounds in order of increasing boiling points:
1-chloropropane, Isopropyl chloride, 1-chlorobutane.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area