Haloalkanes and Haloarenes
Arrange the nucleophilicity (rate of SN2 reactivity) of
(i) H2O, OH–, CH3COO- and CH3O-
(ii) NH3 and PH3.
(i) When the nucleophilic site has the same atom (here an O), nucleophilicity follows basicity. Thus
(ii) When the attacking atoms are different but in the same periodic family, the one with the largest atomic mass is the most reactive. Therefore, PH3 > NH3. This order is the reverse of basicity.
Sponsor Area
Some More Questions From Haloalkanes and Haloarenes Chapter
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
Four isomeric monochlorides.
Draw the structures of major monohalo products in each of the following reactions:![]()
Draw the structures of major monohalo products in each of the following reactions: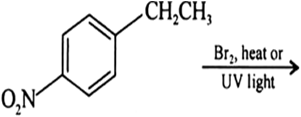
Draw the structures of major monohalo products in each of the following reactions: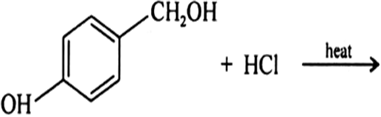
Draw the structures of major monohalo products in each of the following reactions: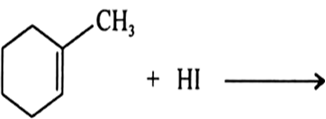
Draw the structures of major monohalo products in each of the following reactions: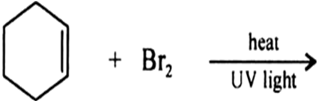
Arrange each set of compounds in order of increasing boiling points:
Bromomethane, Bromoform, Chloro-methane, Dibromomethane.
Arrange each set of compounds in order of increasing boiling points:
1-chloropropane, Isopropyl chloride, 1-chlorobutane.
Arrange each set of compounds in order of increasing boiling points:![]()
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area