Haloalkanes and Haloarenes
Iodination of benzene is carried in the presence of oxidising agent like HIO3. Give reason.
The iodination of benzene is a reversible reaction. Therefore, yield of C6H5I is very poor because HI combines with C6H5I and forms back the reactants.
C6H5 + I2 ⇌ C6H5I + HI
In the presence of oxidizing agent like HIO3 or HNO3, the co-product HI is oxidised to iodine and iodination proceeds favourably in the forward direction.
5HI + HIO3 + 3I2 + 3H2O
Sponsor Area
Some More Questions From Haloalkanes and Haloarenes Chapter
Write structures of different dihalogen derivatives of propane.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i) A single monochloride.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
i)Three isomeric monochlorides.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields:
Four isomeric monochlorides.
Draw the structures of major monohalo products in each of the following reactions:![]()
Draw the structures of major monohalo products in each of the following reactions: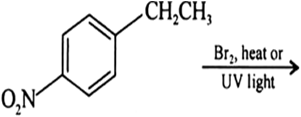
Draw the structures of major monohalo products in each of the following reactions: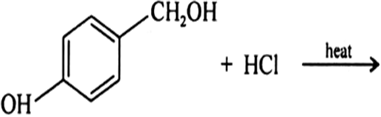
Draw the structures of major monohalo products in each of the following reactions: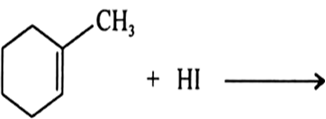
Draw the structures of major monohalo products in each of the following reactions: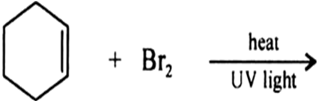
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area