A certain number of spherical drops of a liquid of radius r coalesce to form a single drop of radius R and volume V. If T is the surface tension of the liquid, then
-
Energy = 4VT
 is released.
is released. -
Energy = 3VT
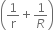 is absorbed
is absorbed -
Energy = 3 VT
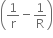 is released
is released -
Energy is neither absorbed nor released.
C.
Energy = 3 VT 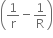 is released
is released
If the surface area changes, it will change the surface energy also.
When the surface area decreases, it means energy is released ad vice-versa.
Change in surface energy is  ... (i)
... (i)
Let, there be n number of drops initially.
So,  ... (ii)
... (ii)
Volume is constant.
So, 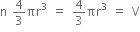 ... (iii)
... (iii)
From equations (ii) and (iii), we have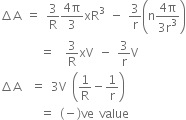
Since, R > r,  A is negative.
A is negative.
That is, the surface area is decreased.
Hence, energy must be released.
Energy released = 
In the above expression, (-)ve sign shows that amount of energy is released.



