What is triple bond or triple covalent bond? Explain the formation of triple bond giving two examples.
The bond formed between two atoms through the sharing of three electron pairs is called a triple bond. Three horizontal lines between two atoms denote a triple bond, e.g., nitrogen molecule is given asN≡N.
(i) Formation of N2 molecule: Nitrogen atoms have five electrons in their outermost shells. Therefore, in N2 molecules, two nitrogen atoms share 3 electrons with each other, thus acquiring the stable structure and forming a N2 molecule with triple bonds.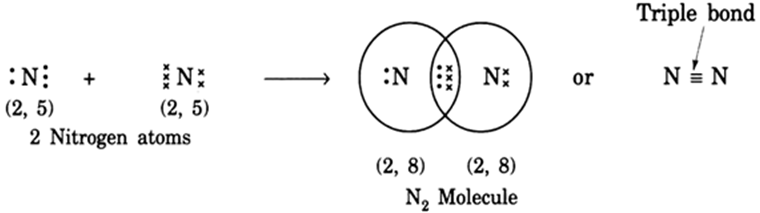
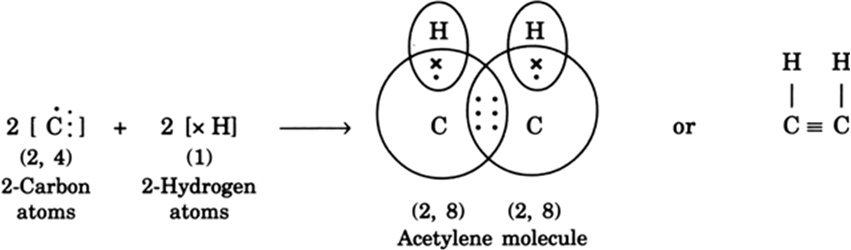
(ii) Formation of acetylene molecule (C2H2): In acetylene molecule, each carbon atom satisfies its valency by sharing one electron with a hydrogen atom and sharing the other three electrons with the other carbon atom as shown: