Question
Explain, how can we find out whether a given substance is a metal if we know its electronic configuration.
Solution
we have the electronic configuration of a substance, we can say whether it is a metal or not. Metals have 1 to 3 electrons in the outermost shell of their atoms.
Sodium, magnesium and aluminium are metals, as these contain 1, 2, 3 electrons respectively in their outermost shells. Their atomic numbers and electronic configurations are as given below:
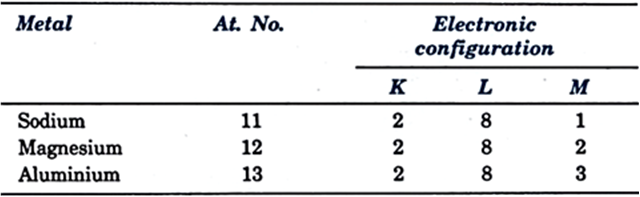
Sodium, magnesium and aluminium are metals, as these contain 1, 2, 3 electrons respectively in their outermost shells. Their atomic numbers and electronic configurations are as given below:




