What are transverse waves? Explain with an example.
Transverse waves are the waves in which particles of the medium vibrate in a direction perpendicular to the direction of wave motion.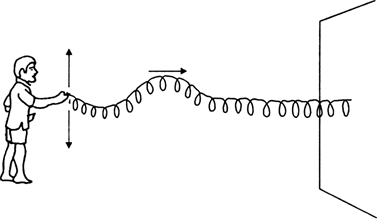
Fig. 12.2 (a). Pulse in a slinky.
i) As shown in the fig. above, take a long slinky and attach it to a wall at its one end.
ii) Give the other end an upward jerk. An upward hump is created in the slinky which travels along the slinky towards the fixed end. Such a sudden disturbance that lasts for short duration is called a pulse.
iii) As shown in Fig. 12.2, if we continuously give up and down jerks to the free end of the slinky, a number of waves begin to travel along the slinky forming a wave train.
iv) Each part of the slinky vibrates up and down while the waves travel along the rope. So the waves in the rope are transverse in nature.
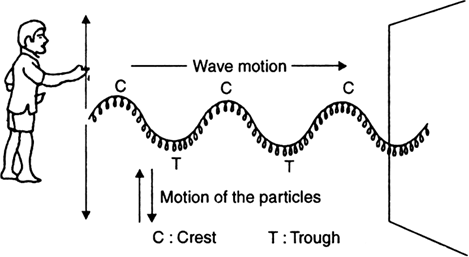
Fig. 12.2 (b). Formation of transverse waves in a slinky.



