Explain how the human ear works.
i) Human ear is a highly sensitive part of the human body which enables us to hear a sound.
ii) It converts the pressure variations in air with audible frequencies into electric signals which travel to the brain via the auditory nerve.
iii) The human ear has three main parts. Their auditory functions are as follows: 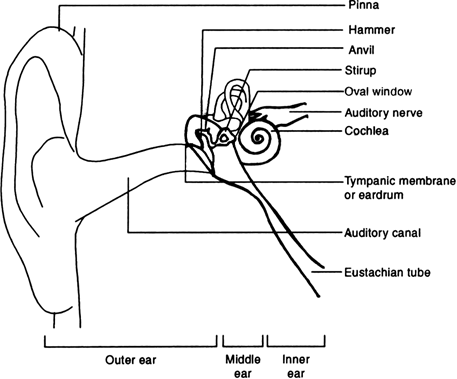
Fig. 12.27. Auditory parts of the human ear
1. Outer ear: The outer ear is called ‘pinna’. It collects the sound from the surrounding. The collected sound passes through the auditory canal. At the end of the auditory canal there is a thin membrane called the ear drum or tympanic membrane.
When compression of the medium produced due to vibration of the object reaches the ear drum, the pressure on the outside of the membrane increases and forces the eardrum inward. Similarly, the eardrum moves outward when a rarefaction reaches. In this way the ear drum vibrates.
2. Middle ear: The vibrations are amplified several times by three bones (the hammer, anvil and stirrup) in the middle ear which act as levers. The middle ear transmits the amplified pressure variations received from the sound wave to the inner ear.
3. Inner ear: In the inner ear, the pressure variations are turned into electrical signals by the cochlea. These electrical signals are sent to the brain via the auditory nerve, and the brain interprets them as sound.



