Describe with the help of a diagram, how compressions and rarefactions are produced in air near a source of sound.
i) Sound travels through air in the form of longitudinal waves.
ii) Consider a vibrating tuning fork producing sound waves as shown in Fig.
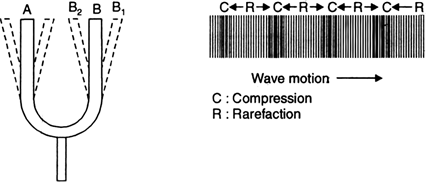
Fig. Tuning fork producing sound waves in air.
iii) The prongs of the tuning fork first move inward and then outwards and so on.
iv) Now, focusing our attention on prong B only, we will see that this prong moves from B to B1 and it compresses the layer of air in front of it.
v) As this compressed layer moves forward, it compresses the next layer and so on. So a wave of compression moves forward. When the prong moves backward from B1 to B2, the pressure of air in the adjoining layer decreases.
vi) The next layer, being at higher pressure tends to move it towards right and so on. So a wave of rarefaction moves forward.
vii) The vibrating tuning fork continues to send a series of compressions and rarefactions.



