Which has more number of atoms, 100 grams of sodium or 100 grams of iron (given atomic mass of Na = 23 u, Fe = 56 u)?
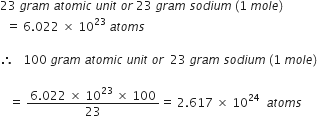
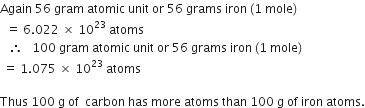
Which has more number of atoms, 100 grams of sodium or 100 grams of iron (given atomic mass of Na = 23 u, Fe = 56 u)?
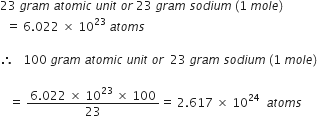
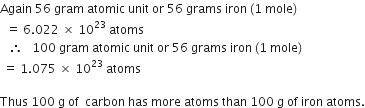
What are polyatomic ions? Give examples.
(a) Magnesium chloride
(b) Calcium oxide
(c) Copper nitrate
(d) Aluminium chloride
(e) Calcium carbonate
Give the names of the elements in the following compounds.
(a) Quick lime
(b) Hydrogen bromide
(c) Baking powder
(d) Potassium sulphate.
Calculate the molar mass of the following substances:
(a) Ethylene
(b) Sulphur molecule, S8
(c) Phosphorus molecule, P4
(d) Hydrochloric acid, HCl
(e) Nitric acid, HNO3.
What is the mass of:
(a) 1 mole of nitrogen atoms
(b) 4 moles of aluminium atoms
(c) 10 moles of sodium sulphite.
Convert into mole
(a) 12 gm of oxygen gas
(b) 20 gm of water
(c) 22 gm of carbon dioxide.
What is the mass of?
(a) 0.2 mole oxygen atoms.
(b) 0.5 mole of water molecules.
Calculate the number of molecules of sulphur (S8) present in 16 gm of solid sulphur.
Calculate the number of aluminium ions present in 0.56 gm of aluminium oxide.
Explain law of conservation of mass.
Mock Test Series