Define the term:Gram molecular mass. How is it related to the mole and Avogadro number?
The molecular mass of a substance (compound) expressed in grams is called its gram molecular mass. Ionic compounds do not contain molecules (they consist of ions) and therefore, molecular masses of ionic compounds are equal to their formula masses.
A gram molecular mass of a compound is equal to the mass of 6.023 x 1023 molecules or 1 mole of the substance.
Examples;
(i) A gram molecular mass of hydrogen molecules (molecular mass = 2)
weighs 2 grams and contains 6.023 x 1023 hydrogen molecules.
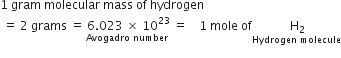
(ii) A gram molecular mass of sodium chloride (molecular or formula mass = 58.5) weighs 58.5 grams and contains 6.023 x 1023 sodium chloride units.



