Calculate the mass in grams of the following:
(i) One mole of sulphuric acid (H2SO4).
(ii) 0.5 mole of aluminium sulphate Al2(SO4)3.
(iii) 0.05 mole of ammonia (NH3).
(iv) 0.7 mole of hydrogen chloride (HCl).
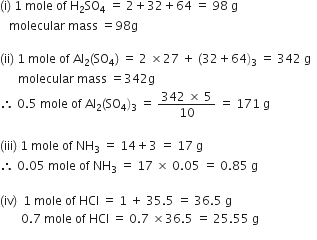
Calculate the mass in grams of the following:
(i) One mole of sulphuric acid (H2SO4).
(ii) 0.5 mole of aluminium sulphate Al2(SO4)3.
(iii) 0.05 mole of ammonia (NH3).
(iv) 0.7 mole of hydrogen chloride (HCl).
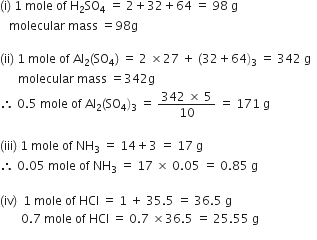
What is the mass of:
(a) 1 mole of nitrogen atoms
(b) 4 moles of aluminium atoms
(c) 10 moles of sodium sulphite.
Convert into mole
(a) 12 gm of oxygen gas
(b) 20 gm of water
(c) 22 gm of carbon dioxide.
What is the mass of?
(a) 0.2 mole oxygen atoms.
(b) 0.5 mole of water molecules.
Calculate the number of molecules of sulphur (S8) present in 16 gm of solid sulphur.
Calculate the number of aluminium ions present in 0.56 gm of aluminium oxide.
Explain law of conservation of mass.
50 g of 10% lead nitrate is mixed with 50 g of 10% sodium chloride in a closed vessel. After the reaction has taken place, it was found that 6.83 g of lead chloride was precipitated. Besides, the reaction mixture contained 90 g water and sodium nitrate. Calculate the amount of sodium nitrate formed.
Name and explain two important laws of chemical combination ?
What are the postulates of Dalton’s atomic theory of matter?
Mock Test Series