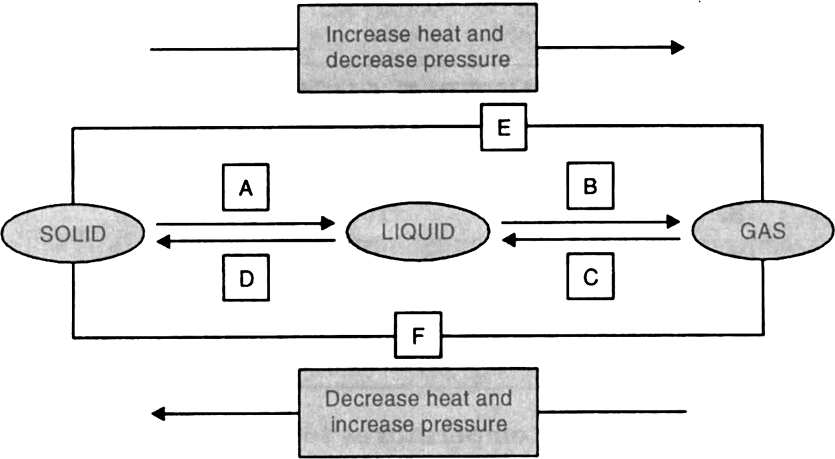
Why is ice at 273K is less energetic than water at the same temperature
Cooling takes place when heat is removed from a system, in case of ice at 273 K, it will take latent heat from the medium to convert itself into water at 273K there will be a change in physical state, whereas in case of water at 273 K there will be no change in state. Hence lesser energy will be taken from the medium.