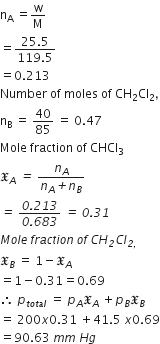Question
Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 25oC are 200 mmHg and 41.5 mmHg respectively, Vapour pressure of the solution obtained by mixing 25.5 g of CHCl3 and 40 g of CH2Cl2 at the same temperature will be
(molecular mass of CHCl3= 119.5 u and molecular mass of CH2Cl2 = 85 u)
-
173.9 mmHg
-
615.0 mmHg
-
347.9 mmHg
-
28.5 mmHg
Solution
A.
173.9 mmHg
Number of moles of CHCl3