Sponsor Area
Solutions
Question
Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL at 370C.
Solution
Solution :
we have given that
Volume of water, V= 450ml=0.450L
Temperature, T = (37+273)K = 310K
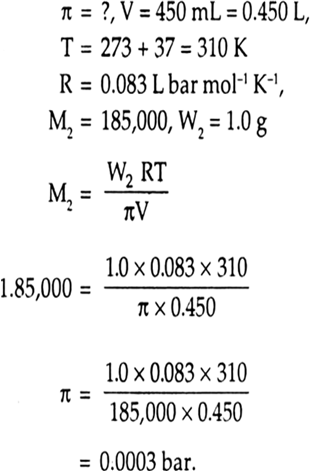
we have given that
Volume of water, V= 450ml=0.450L
Temperature, T = (37+273)K = 310K
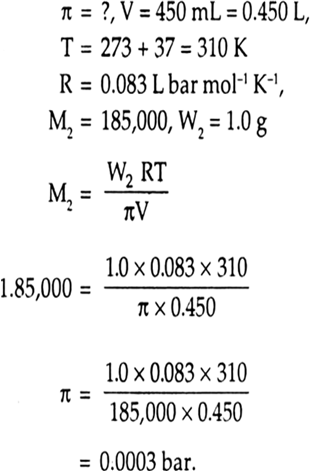
Some More Questions From Solutions Chapter
Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL at 370C.
Suppose a solid solution is formed between two substances, one whose particles are very large and the other whose particles are very small. What kind of solid solution is this likely to be?
Amongst the following compunds identify which are insoluble, partially soluble and highly soluble in water?
(i) phenol (ii) toluene (iii) formic acid (iv) ethylene glycol (v) chloroform, (vi) pentanol.
Two liquids A and B boils at 1450C and 1900C respectively. Which of them has a higher vapour pressure at 800C ?
Sponsor Area
Mock Test Series
Mock Test Series





