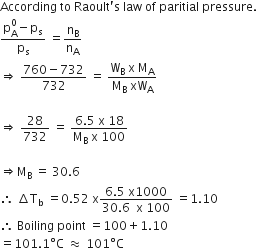Question
A 1000C the vapour pressure of a solution of 6.5 g of a solute in 100 g water is 732 mm. If Kb = 0.52, the boiling point of this solution will be
-
1000C
-
1020C
-
1030C
-
1010C
Solution
D.
1010C