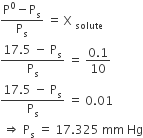Question
The vapour pressure of water at 20oC is 17.5 mm Hg. If 18 g of glucose (C6H12O6) is added to 178.2 g of water at 20oC, the vapour pressure of the resulting solution will be
-
17.675 mm Hg
-
15.750 mm Hg
-
16.500 mm Hg
-
17.325 mm Hg
Solution
D.
17.325 mm Hg