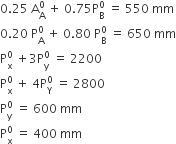Question
Two liquids X and Y form an ideal solution. At 300 K, the vapour pressure of the solution containing 1 mol of X and 3 mol of Y is 550mm Hg. At the same temperature, if 1 mol of Y is further added to this solution, the vapour pressure of the solution increases by 10 mm Hg. Vapour pressure (in mmHg) of X and Y in their pure states will be respectively
-
200 and 300
-
300 and 400
-
400 and 600
-
500 and 600
Solution
C.
400 and 600