Question
On treatment of 100 mL of 0.1 M solution of CoCl3 . 6H2O with excess AgNO3; 1.2 × 1022 ions are precipitated. The complex is
-
[Co(H2O)4 Cl2]Cl.2H2O
-
[Co(H2O)3Cl3].3H2O
-
[Co(H2O)6]Cl3
-
[Co(H2O)5Cl]Cl2.H2O
Solution
D.
[Co(H2O)5Cl]Cl2.H2O
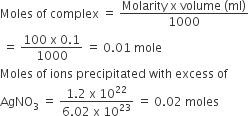
Number of Cl– present in ionization sphere =
It means 2Cl– ions present in ionization sphere
∴ complex is [Co(H2O)5Cl]Cl2.H2O



