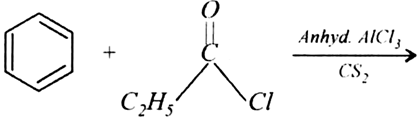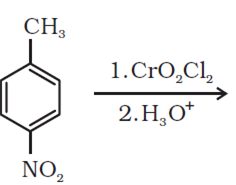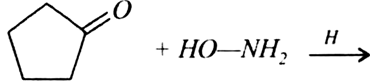Question
One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass of 44 u. The alkene is
-
Propene
-
1-butene
-
2-butene
-
ethene
Solution
C.
2-butene
(a) The general formula of aldehyde compound is CnH2nO. First, calculate the value of n.
(b) Alkene is symmetrical, therefore, only single type of aldehyde is produced as a product.
CnH2nO = 44
CnH2n = 44-16 = 28
n = 2
Therefore, since, the alkenes is symmetrical, then the structure is
CH3 - CH=CH -CH3
Thus, ![]()