Sponsor Area
Solutions
Ethylene glycol is used as an antifreeze in a cold climate. Mass of ethylene glycol which should be added to 4 kg of water to prevent it form freezing at - 6° C will be : (Kf for water = 1.86 K kg mol-1, and molar mass of ethylene glycol = 62g mol-1)
-
804.32 g
-
204.30 g
-
400.00 g
-
304.60 g
A.
804.32 g
ΔTf = iKfm
ΔTf = 6ºC
w1 = mass of ethylene glycol in grams
w2 = mass of solvent (H2O) in grams= 4000g
m1 = Molar mass of ethylene glycol = 62 g
i = 1
i is van't off factor
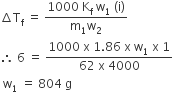
Some More Questions From Solutions Chapter
Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL at 370C.
Suppose a solid solution is formed between two substances, one whose particles are very large and the other whose particles are very small. What kind of solid solution is this likely to be?
Sponsor Area
Mock Test Series
Mock Test Series





