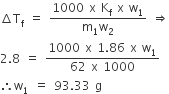Question
Kf for water is 1.86K kg mol–1.If your automobile radiator holds 1.0 kg of water, how many grams of ethylene glycol (C2H6O2) must you add to get the freezing point of the solution lowered to –2.8°C?
-
72 g
-
93 g
-
39 g
-
27 g
Solution
B.
93 g
Coolant is glycol (C2H6O2) and is non-electrolyte.
ΔTf =2.8°