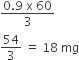Question
3 g of activated charcoal was added to 50 mL of acetic acid solution (0.06N) in a flask. After an hour it was filtered and the strength of the filtrate was found to be 0.042 N. The amount of acetic acid adsorbed (per gram of charcoal) is:
-
18 mg
-
36 mg
-
42 mg
-
54 mg
Solution
A.
18 mg
The initial strength of acetic acid = 0.06N
Final strength = 0.042 N
Volume given = 50 mL
there Initial m moles of CH3COOH
= 0.06 x 50 = 3
Final m moles of CH3COOH
= 0.042 x 50 = 21
therefore, m moles of CH3COOH absorbed
= 3-2.1
= 0.9 m mol
Hence, mass of CH3COOH absorbed per gram of charcoal
=