The reaction of propene with HOCl(Cl2+H2O) proceeds through the intermediate:
-
CH3−CH+−CH2−Cl
-
CH3−CH(OH)−C+H2
-
CH3−CHCl−C+H2
-
CH3−C+H−CH2−OH
A.
CH3−CH+−CH2−Cl
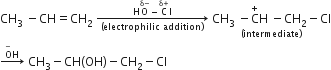
The reaction of propene with HOCl(Cl2+H2O) proceeds through the intermediate:
CH3−CH+−CH2−Cl
CH3−CH(OH)−C+H2
CH3−CHCl−C+H2
CH3−C+H−CH2−OH
A.
CH3−CH+−CH2−Cl
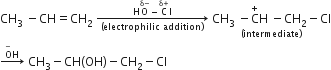
Draw the structures of major monohalo products in each of the following reactions:![]()
Draw the structures of major monohalo products in each of the following reactions: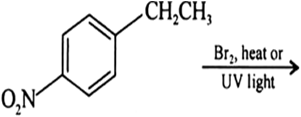
Draw the structures of major monohalo products in each of the following reactions: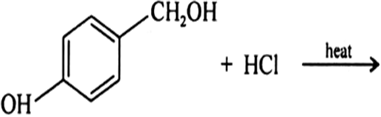
Draw the structures of major monohalo products in each of the following reactions: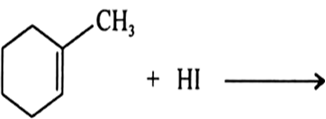
Draw the structures of major monohalo products in each of the following reactions: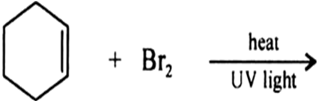
Arrange each set of compounds in order of increasing boiling points:
Bromomethane, Bromoform, Chloro-methane, Dibromomethane.
Mock Test Series