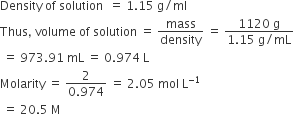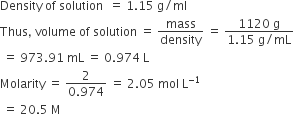Question
The density of a solution prepared by dissolving 120 g of urea (mol. Mass = 60 u ) in 1000g of water is 1.15 g/mL. The molarity of this solution is
-
0.50 M
-
1.78 M
-
1.02 M
-
2.05
Solution
D.
2.05
Total mass of solution
= 1000 g water +120 g urea
= 1120 g