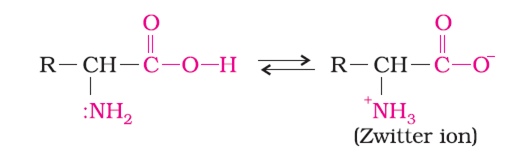Amino acids show amphoteric behaviour. Why?
In aqueous solution, the carboxyl group of an amino acid can lose a proton and the amino group can accept a proton to give a dipolar ion known as zwitter ion.
Therefore, in zwitter ionic form, the amino acid can act both as an acid and as a base. Thus, amino acids show amphoteric behaviour.