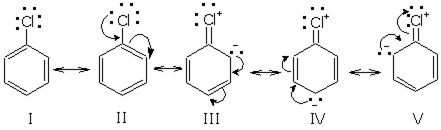
Out of chlorobenzene and benzyl chloride, which one gets easily hydrolysed by aqueous NaOH and why?
Benzyl chloride gets easily hydrolysed by aqueous NaOH because chlorobenzene a partial double bond develops between carbon and chlorine bond due to which bond become short and strong substitution of chlorine become very difficult.