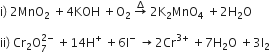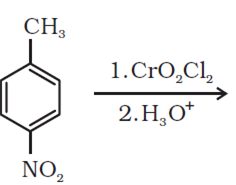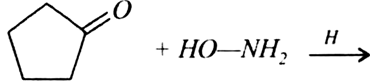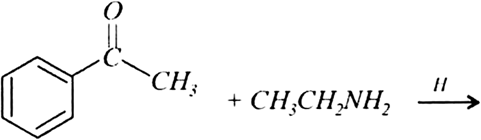i) Mn Shows the highest oxidation state of +7 with oxygen but with fluorine it shows the highest oxidation state of +4.
ii) Cr2+ is a strong reducing agent.
iii) Cu2+ salts are coloured while Zn2+ salts are white.
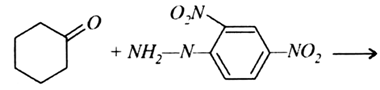
b) Complete the following equations:
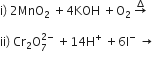
i) Mn Shows the highest oxidation state of +7 with oxygen but with fluorine, it shows the highest oxidation state of +4 because of the ability of oxygen to form multiple bonds with Mn metal.
ii) Cr2+ is strongly reducing in nature. It has a d4 configuration. Cr2+ is a stronger reducing agent because it can lose one of its electrons to become Cr3+ in which the t2g level of d-orbital is half filled and the eg level is empty.which is a more stable configuration.
iii) The electronic configuration of Zn = 3d10 4s2
Zn2+ = 3d10
where as the electronic configuration of Cu = 3d10 4s1
Cu2+ =3d9
In the case of Zn fully filled d orbital is present therefore no d-d transition can be possible in this case and it is colourless.
In the case of copper 3d9 because of d-d transition electrons emits light in the visible range and hence they are coloured compounds.
b)