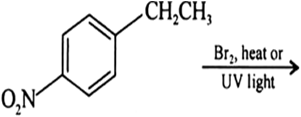
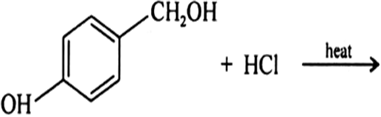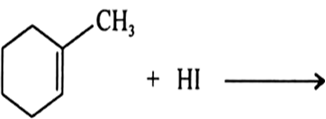(a) Draw the structures of major monohalo products in each of the following reactions :
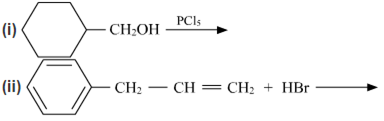
(b) Which halogen compound in each of the following pairs will react faster in SN2 reaction:
(i) CH3Br or CH3I
(ii) (CH3)3C−Cl or CH3−Cl
a)
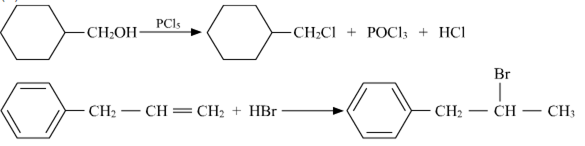
b)
(i) CH3I will react faster in SN2 reaction than CH3Br. This is because I− is a better leaving group, owing to its greater size than Br−. As a result, it will leave at a faster rate in the presence of an incoming nucleophile.
(ii) CH3−Cl will react faster in SN2 reaction than (CH3)3 C−Cl, as CH3−Cl is a primary halide whereas (CH3)3C−Cl is a tertiary halide. Primary halides undergo SN2 reactions faster.