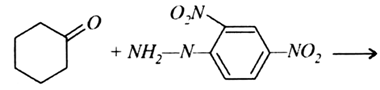
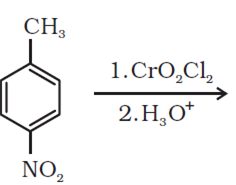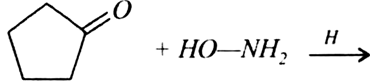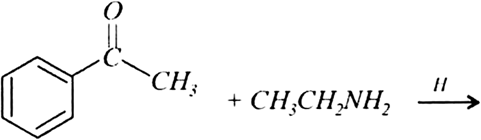(a) Write the products of the following reactions:
(b) Which acid of each pair shown here would you expect to be stronger?
(i) F — CH2 —COOH or Cl — CH2 — COOH
(ii) 
(a)
(i)
![]()
This reaction is Clemmensen reduction.
(ii)

This reaction is Rosenmunds reduction.
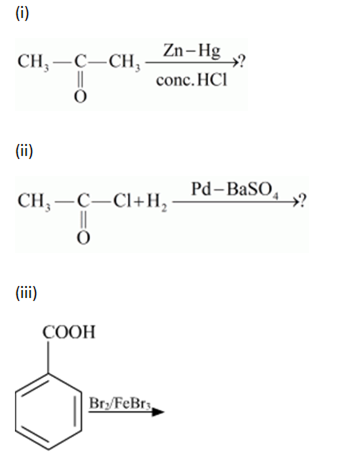
(iii)
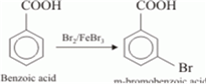
This reaction is an electrophilic substitution reaction.
(b)
(i) F — CH2 — COOH is a stronger acid than Cl — CH2 — COOH because F is more electronegative than Cl so it will favour the release of H+ ion faster by dragging the electron density towards itself more as compared to Cl.
(ii) Acetic acid is a stronger acid than phenol. On losing a proton, carboxylic acid forms carboxylate ion and phenol forms phenoxide ion. The negative charge is delocalized in both the molecules. The conjugate base of the carboxylic acid has two resonance structures (shown below) in which negative charge is delocalized over two oxygen atoms which stabilises the carboxylate ion.
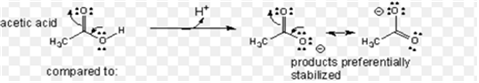
On the other hand, in phenoxide ion, the negative charge is delocalized over entire molecule on the less electronegative carbon atom as given below.
Thus resonance in phenoxide is not important as compared to resonance in carboxylate ion.
Further, in carboxylate ion, the negative charge is effectively delocalized over two oxygen atoms whereas it is less effectively delocalized over one oxygen atom and the less electronegative carbon atom in phenoxide ion.
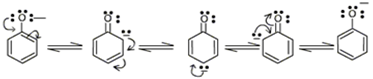
Thus acetic acid is a stronger acid than phenol.