Question
Out of 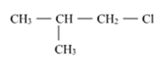 and
and 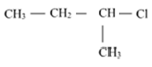 which is more reactive towards
which is more reactive towards
SN 1 reaction and why?
Solution
In the SN1 reaction the formation of carbocation is the rate determining step, and also the stability of carbocation would determine its reactivity.
The order of stability of carbocation is given as Tertiary>Secondary> Primary> methyl.
Here, 1-chloro-1-methylpropane would form a secondary carbocation, while 1-chloro-2- methylpropane would form a primary carbocation, which is less stable than secondary carbocation. Hence, reactivity towards the SN1 reaction would be higher for 1-chloro-1- methylpropane.



