Answer the following question:
(i) What is meant by the chirality of a compound? Give an example.
(ii) Which one of the following compounds is more easily hydrolyzed by KOH and why?
CH3CHCICH2CH3 or CH3CH2CH2Cl
(iii) Which one undergoes S N 2 substitution reaction faster and why?
![]()
(i)Chirality: It is a geometrical property of a rigid object ( or molecules ) by which it gets such spatial arrangement of points or atoms that, the molecule becomes non-super imposable of its mirror image.
The chiral molecule of the object does not have any element of symmetry like, a mirror image, centre of inversion (i) etc.
3-bromopent-1-ene is represented as
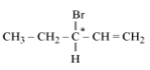
The centre of chirality is designated as *. It is due to the presence of four different groups.
(ii) Due to +I effect of alkyl groups the 2° carbonium ion CH3—CH+ —CH2—CH3 derived from sec. butyl chloride is more stable than the 1° carbonium ion CH3—CH2—C H2+ derived from n-propyl chloride. Therefore sec. butyl chloride gets hydrolyzed more easily than n-propyl chloride under SN1 conditions.
(iii) ![]() Undergoes SN2 substitution reaction faster.
Undergoes SN2 substitution reaction faster.



