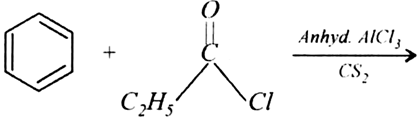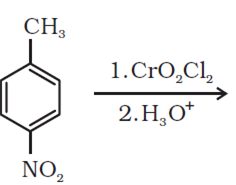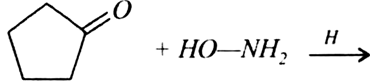Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Benzaldehyde and Benzoic acid
(ii) Propanal and Propanone
Benzaldehyde and Benzoic acid
Test-1 Through sodium bicarbonate Benzaldehyde does not react with sodium bicarbonate. However, benzoic acid will produce brisk effervescence on reaction with sodium bicarbonate as shown in the given reaction:
C6H5COOH + NaHCO3 ---> C6H5COONa + H2O + CO2
Test-2 Through, Tollen's reagent Benzaldehyde reacts with an ammoniacal solution of silver nitrate to form a silver mirror.
C6H5CHO + 2[Ag(NH3)2]+ + 3OH- --> C6H5COO- + 2Ag + 2H2O + 4NH3
However, no such reaction is given by benzoic acid.
(ii) Test-1 Iodoform Test
Propanone gives positive iodoform test, as it contains CH3CO group, whereas propanal does not give iodoform test. The reaction is as follows:
![]()