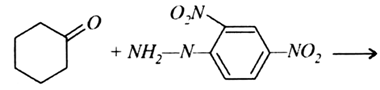
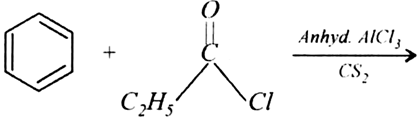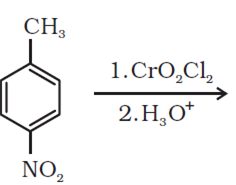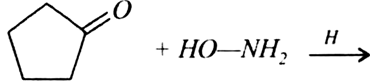Question
Account for the following:
Carboxylic acids with five or less carbons are water soluble, but many with six or more carbons dissolves in alcohols.
Solution
RCOOH dissolves in water because COOH of RCOOH can H-bond with water in two ways.
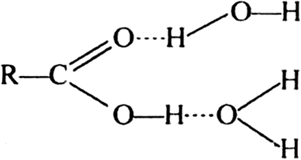
The R group is non-polar and hydrophobic and this effect dominates when R possess more than five carbon. Thereby, decreasing its solubility in more polar solvent as water, but the solubility in less polar solvent such as alcohol increases.

The R group is non-polar and hydrophobic and this effect dominates when R possess more than five carbon. Thereby, decreasing its solubility in more polar solvent as water, but the solubility in less polar solvent such as alcohol increases.