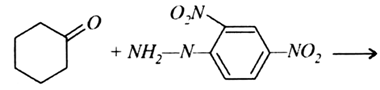
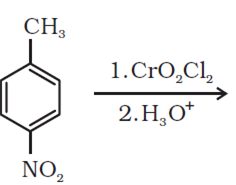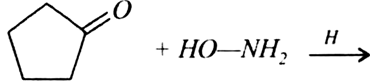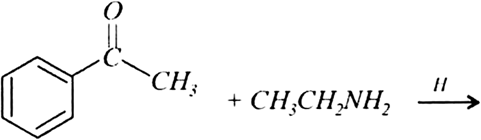An organic compound (A) (Mol. formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). Write equations for the reactions involved.
The given molecular formula C8H16O2 gives carboxylic acid (B) and an alcohol (C) on hydrolysis with H2SO4. Thus, compound may be an ester. Further, alcohol C gives acid B on oxidation with chromic acid.Thus, B and C must contain equal number of carbon atoms. As given compound contain 8 carbon atom, each B and C must contain 4 atom. On dehydration, alcohol C gives but-2-ene. Therefore, C is of straight chain and hence, it is butan-1-ol.
On oxidation, butan-1-ol givers butanoic acid. Hence, acid B is butanoic acid. Therefore the given compound is ester is butylbutanoate.
![]()
The above statement is explained as,